To draw all resonance structures take the lewis structure we drawn by using VESPR rule. 6 Draw all significant resonance structures for the following compound.
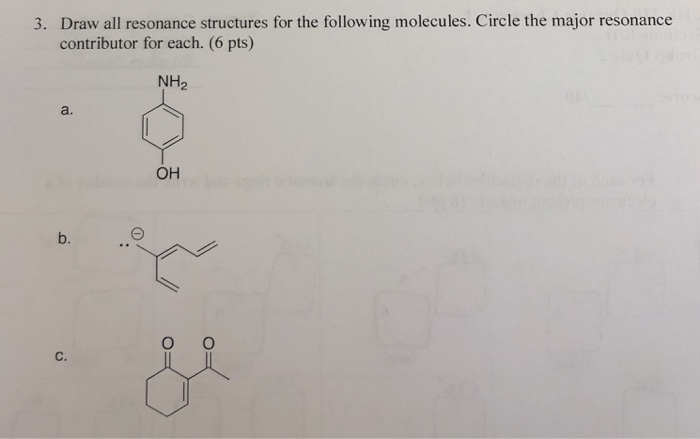
Solved 3 Draw All Resonance Structures For The Following Chegg Com
Organic Chemistry 222 Fall 2016 Recitation Problem Set 2 Sep 13th 11 a Use resonance structures to help you identify all sites of low electron density d in the following compound.

. Resonance structures are a set of two or more Lewis Structures that collectively describe the. As a result the C-H bond is almost completely broken in the transition state and the carbon atom has significant radical. The first thing we should always do anytime we do residences drawing all of our lone pairs.
This resonance hybrid is illustrated below. Answer on next slide. Count the electrons in the valence shell to check if the octet is completed.
O Step 1 Your answer is incorrect First add curved arrow s to show the resonance using the following patterns a pi bond between two atoms of differing electronegativity Modify the second structure given to draw the new resonance. Weve got the study and writing resources you need for your assignments. Lets draws many residents structures as possible for the following species.
Resonance is an exercise within a molecule following the Valence Bond Theory of bonding that defines the delocalization of electron. Draw significant resonance structures for the following. Start your trial now.
First week only 499. Assessing the Relative Importance of Resonance Structures. Two must-follow rules when drawing resonance structures.
Draw Significant Resonance Structures For The Following Compound. Resonance structures are a set of two or more Lewis Structures that collectively describe the. B Use resonance structures to help you identify all sites of high electron density d- in the.
Show the electron shift using curved arrow notation. The carbon atom needs one more electron and each oxygen atom needs one more electron to complete the octet. For each of the following compounds draw all of the significant resonance structures.
O First add curved arrow s to show the resonance using the following pattern. Start your trial now. Organic Chemistry as a Language.
We want to draw all of the president structures of this eye on here. IMAGE IS NOT AVAILABLE TO COPY Answer. The single bond is active by default.
The net charge on the central atom remains 1. Double bond double bond. Include lone pairs and charges in your structure.
So this oxygen should have two lone pairs since its neutrally charged and has two bombs to it. Combine each atom with a single bond to the central atom by contributing one electron from each atom for the bond. Oh mhm ch two minus.
All the following resonance structures that bear charge are the sites that have low electron density. This problem has been solved. This goes in here.
This double bond comes out here you would have h 3 cc oh minus double bond ch two for be Gods six member ring. Modify the second structure given to draw the new resonance structure. CH CH HJC Draw the molecules on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolbars.
Fill in any missing formal charges. A Draw all significant resonance structures for the following compounds. Include lone pairs and charges in your structure.
And since this carbon over has a negative charge on it it should have. Solution for draw all significant resonance structures. Which will favor a reaction to make products.
First week only 499. IMAGE IS NOT AVAILABLE TO COPY Answer. Draw the resonance structure of the following intermediates in the nitration of phenol.
A Draw the resonance arrows to form the structure on the right b Circle the major resonance. The more resonating structures a molecule or ion has the more stable it becomes. View the full answer.
Resonating structures represent the movement of electron clouds inside a molecule when it has alternating double bonds. Step 1 Get help answering Molecular Drawing questions First add curved arrows to show the resonance using the following pattern. O-N OH H CH.
The resonance structure with the Formal Charge closest to zero is the most accepted structure however the correct Lewis structure is actually a combination of all the resonance structures and is not solely describe as one. An allylic lone pair. Start your trial now.
But to identify each resonance structures it is good to show arrows. Identify the weakest C-H bond in the following compound. The second-row elements C N O F can only handle up to.
Draw resonance structures for each of the following compounds. Modify the second structure given to draw the new resonance structure. A Draw all significant resonance structures for the following compounds.
Draw significant resonance structures for the following compound. The three possible resonance structures of NO 3 are illustrated below. The transformation given is Q.
For a with H 3 cc double bond. First week only 499. Join our telegram group for regular updates and free study material httpsifttt3cmV3NY.
If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above the partial charge on each oxygen atom will be equal to - ⅔. Rules for drawing resonance structures. Draw two additional resonance structures for the following compound.
Solution for Draw significant resonance structures for the following compound. 2 Do not break single bonds. Ii CH2 CH - CH CH2 iii CH2 CH - H C O.
Draw all significant resonance structures for the following compound. Organic Chemistry as a Language. For each of the following compounds draw all of the significant resonance structures.
In resonance structures it does not require to show transformation of electrons by arrows. Click hereto get an answer to your question Draw the resonance structures of the following compounds. I dont wanna put another negative but it should have a.
I CH2 CH - Cl. Assessing the Relative Importance of Resonance Structures. Draw two additional resonance structures for the following compound.
Chemistry questions and answers. Weve got the study and writing resources you need for your assignments. 1 Do not exceed the octet on 2nd-row elements.
There are also several patterns for drawing resonance structures of radicals. In following examples arrows are used to show electrons transformation. Solution for Draw significant resonance structures for the following compound.
Draw 3 significant resonance structures for the compound shown below. We have some lone pairs resonance structure here. Draw resonance structures for following compounds Show electron shift using curved arrow notation Draw the resonance structures for the following compounds.
An allylic lone pair.

Solved Draw Significant Resonance Structures For The Chegg Com

Leaving Groups Chemwiki Organic Chemistry Chemistry Organic Chem
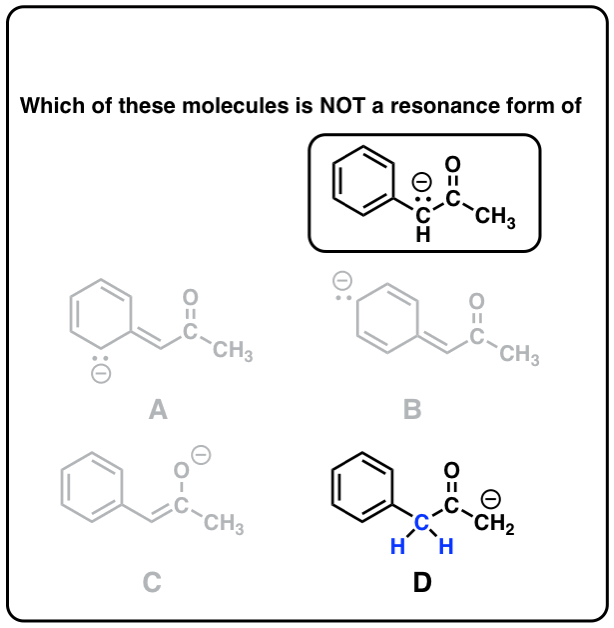
Resonance Structures 4 Rules On How To Evaluate Them With Practice
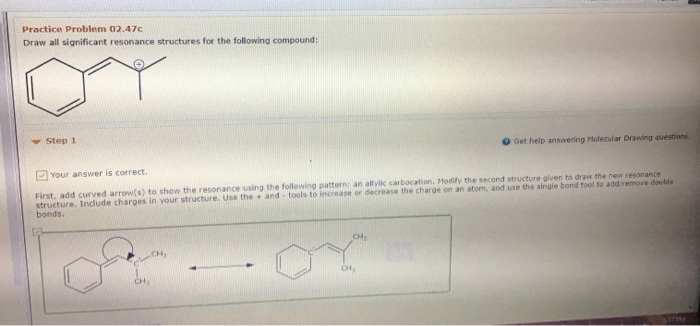
Solved Practice Problem 02 47b Draw All Significant Chegg Com

Construction Of The Lewis Electron Dot Structure Of Cn2h2 Lewis Electrons Dots

Bha And Bht In Your Food What Are They Bht Bha Peace Symbol

Resonance Structures Chemistry Steps Chemistry Octet Rule Fun To Be One
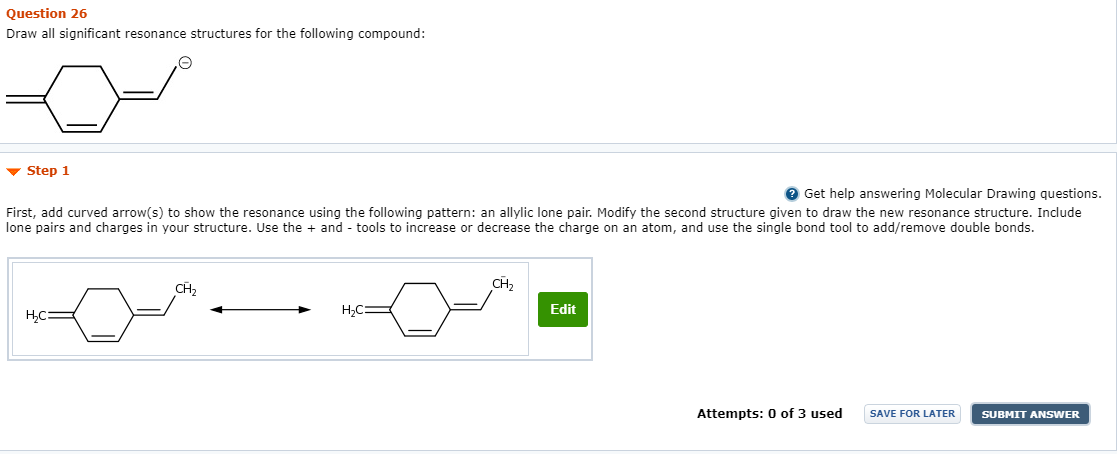
Solved Question 26 Draw All Significant Resonance Structures Chegg Com
0 comments
Post a Comment